“Osteoarthritis is the most common form of arthritis, affecting millions of people worldwide. It occurs when the protective cartilage that cushions the ends of the bones wears down over time. Although osteoarthritis can damage any joint, the disorder most commonly affects joints in your hands, knees, hips and spine. Osteoarthritis symptoms can usually be managed, although the damage to joints can’t be reversed.”
The Mayo says the only thing that those who have osteoarthritis can do is stay active, maintain a healthy weight, and try treatments to slow the progression of the disease while helping to improve pain and joint function.
Bone and Joint Canada (BJC), a not-for-profit organization that racks osteoarthritis notes that 4.6 million out of 40 million, that is more than 10% of the population, suffer from osteoarthritis. And that number is expected to grow in thirty years to 10 million.
In the United States, the Centers for Disease Control have calculated that osteoarthritis affects 32.5 million, and as in Canada, that number is expected to grow as the population of the country ages.
No country on the planet, in fact, is spared because osteoarthritis seems to come with aging. But is the Mayo Clinic right? Is osteoarthritis uncurable?
University of Adelaide researchers in Australia where one in five over the age of 45 suffer from the condition, beg to disagree with the Mayo. These researchers are working to restore Grem1 stem cells which disappear as we age coincident with cartilage thinning and degeneration.
A paper published in Nature Communications on October 31, 2023, describes the efforts of the research being done at the University’s Medical School. It describes osteoarthritis as the dysfunction of mature cartilage cells combined with an imbalance of Grem1 stem progenitor cells. Dr. Jia Ng from the Adelaide Medical School, is the lead author of the paper. In a news release from the University, he states:
“The findings of our study reimagine osteoarthritis not as a ‘wear and tear’ condition but as an active, and pharmaceutically reversible loss of critical articular cartilage stem cells.” With the discovery of the role of Grem-1 stem cells related to cartilage thinning, “are now able to explore pharmaceutical options to directly target the stem cell population that is responsible for the development of articular cartilage and progression of osteoarthritis.”
Research with stem cell injections so far has not effectively repaired osteoarthritis. A five-year clinical trial using Fibroblast Growth Factor 18 (FGF18), encoded by the FGF18 gene in humans and first discovered in 1998, has shown promise. A current Phase 3 trial using Sprifermin, a recombinant human FGF19 analog, has been encouraging.
Until recently regenerating cartilage medically has been next to impossible. Surgical efforts have been tried. These include:
- Drilling holes in bones to stimulate natural healing and cartilage regrowth.
- Inducing small injuries in bones within a joint to stimulate self-healing.
- Membrane-induced Autologous Chondrocyte Implantation or MACI which harvests chondrocytes from a patient, grows the cells in the lab, and then surgically implants them into damaged areas.
- Bone and cartilage grafting takes a larger segment of material from the patient to implant it where it is needed.
- Mosaicplasty involves harvesting healthy cartilage from non-weight-bearing areas in a joint and transplanting it into the damaged area.
A pharmaceutical solution would be a welcome change and could prove the Mayo Clinic’s pronouncement about osteoarthritis being irreversible false.
]]>Before I underwent knee replacement surgery three years ago last July, I was hellbent on waiting for a stem cell therapy alternative to regrow my arthritic joint. Having endured more than a decade of discomfort and a course of cortisone injections to keep the surgeons at bay, the advances in stem cell research had not yet reached a point where it could be considered a non-surgical alternative therapy.
There were several clinical trials. The closest still accepting volunteers was in Chicago. The only one in Toronto had reached its complement of candidates. Eventually, I was referred to an orthopaedic surgeon who explained to me why knee replacement using stem cells to regrow cartilage was still seeking answers for its most challenging limitation.
Stem Cells Used to Treat a Gorilla with Osteoarthritis
Mesenchymal stem cells harvested from a patient could be turned into chondrocytes, the name given to cartilage cells. Cartilage cells, however, need a matrix upon which to grow. That natural matrix is made of collagen, a structural protein our bodies produce that forms connective tissue. At the time of my referral, no stem cell treatment had yet to come up with a way to produce collagen and put it where it was needed to support growing cartilage.
The treatment of Liesel, the elderly gorilla, used mesenchymal stem cells harvested from a younger donor female gorilla who had undergone minor surgery in 2022. StemCellX, a company formed by leading stem cell scientists, worked with researchers from the University of Sheffield in the United Kingdom on Liesel’s treatment.
StemCellX is focused on cutting-edge research and stem cell treatments for veterinary medicine. It provided the harvested mesenchymal stem cells that were kept frozen until the procedure. There was no mention of concern about Liesel rejecting the stem cell infusion. That’s because mesenchymal cell transplants exhibit low rejection rates and tend to be well tolerated in transplantation. There was high confidence that Liesel would benefit since StemCellX has successfully treated dogs and horses with moderate to advanced osteoarthritis.
Human Clinical Stem Cell Trials for Osteoarthritis Show Success
Several clinical trials using mesenchymal stem cell therapies for treating osteoarthritis have recently reported success. One from Brazil documents the use of bioscaffolds made up of collagen or hyaluronic acid applied to knee joints in place of natural collagen with intra-articular injections of mesenchymal stem cells to induce cartilage regeneration in osteoarthritic knee joints. The following illustration appears in the journal article that describes this clinical trial.
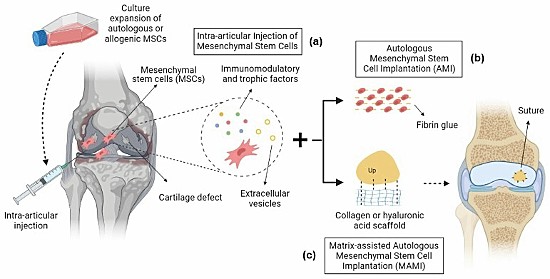
In another two-year case study published in the journal, Regenerative Medicine, 329 patients with knee osteoarthritis received mesenchymal stem cell therapy with significant improvements to the knee joint. In this clinical trial, the goal was to reduce or delay the need for total joint replacement.
Treating Parkinson’s Disease with Embryonic Stem Cells
In a University of California, Irvine (UC Irvine) clinical trial, laboratory-made neurons grown from embryonic stem cells have been injected into the brains of 12 patients with Parkinson’s. The clinical trial is ongoing but initial results are encouraging showing that the added neurons have formed synapses to link to the patients’ brain cells and produce dopamine.
Dopamine is a neurotransmitter used to send signals to neurons in the brain. Naturally produced by our bodies, too much dopamine causes aggressive behaviours in people Too little leads to diseases like Parkinson’s, restless leg syndrome and depression. Dopamine is often called the pleasure molecule because when produced it can induce those types of feelings. Getting the dopamine balance right, therefore, has turned out to be a way to treat Parkinson’s.
In the UC Irvine study, PET scans have been used to observe how the brain’s uptake of the neurons is succeeding. After a year, these laboratory-produced neurons appear to be surviving while Parkinson’s symptoms are shown to be lessening. The university’s ongoing research into Parkinson’s is not only focused on delaying the onset of the disease but also rebuilding the brains of patients suffering from it. The ongoing trial which started in May 2021 will conclude in 2024. At that time and based on reported success, the plans are to increase the number of Parkinson’s patients receiving neurons created from embryonic stem cells.
In the United States, embryonic stem cell research is considered highly controversial. Those opposed to a woman’s right to choose associate this type of research with abortion with the harvesting and use of embryonic stem cells violating the right to life and equivalent to committing murder. UC Irvine’s embryonic stem cell research is possible because the State of California is funding this important field of study, recognizing that scientific progress in this field could inevitably improve the quality of life for millions including Parkinson’s Disease sufferers.
]]>Hai Zhang, a professor of restorative dentistry at the University, along with several colleagues has found a way to generate ameloblasts. What are they? Ameloblasts are one of two cells that exist in human embryos responsible for the formation of our teeth. The other cells are called odontoblasts—the former secrete enamel, the latter dentin.
The process of tooth development is called odontogenesis. The two cells mentioned above are critical to tooth formation. Enamel keeps our teeth surfaces hard and strong throughout our lifetimes. Mineralization of teeth begins early in embryonic development. Dentin precedes enamel production, both critical to giving us a healthy set of choppers.
But life has its travails. What we eat and chew causes the enamel surfaces of our teeth to wear thin, crack and decay. And because it is embryonic cells that form the enamel and then die out once our teeth have been produced, we have no biological means to repair them.
Researchers decided to take on the task of trying to create these lost before-birth enamel-producing ameloblasts. To do they used single-cell combinatorial indexing RNA sequencing or sci-RNA-seq. This technique reveals which of the genes in a cell are active in sequence during development.
The RNA molecule is the letter carrier of the cell while genes are the letter writers. Hence the term messenger RNA or mRNA (a term for which many of us have become more familiar from mRNA COVID vaccines), the molecule that carries the instructions to assemble proteins which serve as the letter reader and construction crew in our analogy. Changes om levels of mRNA differ during stages of embryonic cell development which determines which of our genes are sequentially activated.
What the University of Washington researchers were able to do is track each gene activation stage and create a roadmap to develop ameloblasts from undifferentiated stem cells using a software tool called Monocle. The software was able to predict the biochemistry sequence to test on stem cells. They exposed the cells using computer-designed proteins and chemicals. The latter activated different genes in sequence to create the final roadmap. While doing the work on ameloblasts they uncovered another cell type, subodontoblasts which may precede the development of odontoblasts.
The end result was the stem cells were induced to create multicellular mini-organs called organoids which resembled the structures seen in developing human teeth. Now the team is hoping to improve the process to make enamel as durable as that found in a healthy mouth and develop methods for applying the technique to fill cavities and do other enamel restoration. Imagine a future where we have stem cells activated to fill cavities, do other enamel repair, and even replace lost teeth.
The results of the current project were published last week in the science journal, Developmental Cell.
]]>Before surgery, I had been dealing with osteoarthritis in both knees for almost two decades. My left knee was far worse than the right. I had been receiving cortisone injections regularly to keep me ambulatory. But in July 2020 I had a prosthetic joint implanted.
For years I had put off the surgery in the hope that a less invasive therapy using stem-cell-generated cartilage would become available. It is cartilage that provides cushioning to allow the knee joint to work without bone-on-bone contact. In osteoarthritis, the cartilage wears away until nothing is cushioning the immense stresses and loads that knees take on during daily activities.
As a surgeon explained to me, the reason stem-cell therapies to repair knees has yet to succeed isn’t that we cannot generate cartilage from stem cells, it is the collagen found in knee joints that cannot be restored or regrown. You may be familiar with collagen because of the many beauty supplements and treatments being sold on the market today. The collagen used for these isn’t the same as collagen found in knee joints. The complex collagen proteins in the knee are very different and play an important role in cartilage maintenance. Although we can generate chondrocytes (cartilage from stem cells), producing collagen suitable for knee therapies this way has yet to be done successfully.
So what research into non-surgical alternatives is being worked on today? An article entitled “Adhesive hydrogels in osteoarthritis: from design to application” appeared in the Springer Nature journal, Military Medical Research on January 30, 2023. It described research into developing hydrogels for osteoarthritic knee joint repair. Either natural or synthetic, the adhesive characteristics of hydrogels could be an answer.
Can Hydrogels Do The Work of Collagen?
As previously stated, hydrogels have great adhesive characteristics making them a good collagen substitute when attempting to regrow and restore cartilage in knee joints. The hydrogel material infiltrates the pores and irregularities found on tissue surfaces. This characteristic is called mechanical interlocking and it produces flat, slippery and firm surfaces. Injecting a layer of chondrocytes after hydrogels have bonded with the knee joint’s bone surfaces could become a potential non-surgical treatment. The problem, however, is that although the hydrogels bond to the bone, so far, getting the cartilage cells to do this has been another story.
A range of different adhesive hydrogels is being evaluated. The ideal is a hydrogel with double-sided adhesive characteristics to bond both bone and cartilage. Some are composed of contrasting structures. These are referred to as having double-network characteristics, elasticity on one side and stiffness on the other. Current research is looking at bacterially-engineered hydrogels coming from natural sources such as barnacles, mussels and sandcastle worms.
A material challenge for hydrogels will need to be tested when hardening. When used on knees they cannot be too brittle or too soft. The hydrogels have to stand up to the humid environment that characterizes our knees and bodies (we are mostly water). They have to respond to wear and tear caused by enormous stresses put on knee joints. And they have to be applied to the joint in the least invasive way possible. That’s a lot of issues still to be sorted.
The authors, in concluding their paper, summarize what future hydrogel-based treatments must provide:
- Material characteristics that make bone and cartilage bond and work under various biochemical and environmental conditions aimed at rigid integration and mechanical stability.
- Biological functionality equivalent to a healthy knee with performance and durability characteristics better than any knee prosthetic.
When I started my search for an alternative treatment for my osteoarthritis, it was more than a decade ago as I read and learned about stem cells, chondrocytes and collagen. And it likely will be another decade before non-surgical knee repairs using the techniques and materials described above become mainstream. The solution needs a multidisciplinary approach involving chemistry, biology, pharmaceuticals and significant clinical trials.
]]>Peter goes on to describe how this seamlessly impossible capability is much closer to reality as he discusses work by two different research teams working with synthetic mouse embryos created from stem calls rather than eggs and sperm, and about keeping the embryos alive for almost the gestational period for mice using artificial wombs. These embryos developed beating hearts and brain stems.
The question of why researchers are doing this is fundamental to Peter’s enthusiasm for this work. He sees it as giving us new medical insights into embryology, an alternative to infertility in parents trying to conceive, and the potential to grow organs that would not be rejected in transplants.
What follows are Peter’s words with a few edits from me. I’d be interested in hearing back from readers about this new scientific endeavour.
Nature’s Black Box
How life evolves from a single fertilized cell to an organism with hundreds of billions or trillions of differentiated cells is a fundamental question scientists are only just beginning to understand. Developmental biologists have made significant progress studying animals whose young develop in transparent eggs, such as zebrafish and frogs. But mammalian development is harder to observe because it takes place inside a uterus. Ethical concerns and tight regulations complicate research on human embryos even further.
In recent years, there has been progress coaxing embryonic stem cells into structures resembling blastocysts, the precursor of embryos, which provide an essential model for early human development that sidesteps some of these issues. But without the supportive environment of the womb, there’s only so far these so-called “blastoids” can survive, and have the chance to develop into the later stages of embryonic development.
Artificial Wombs and Artificial Procreation
In August of 2022, a pair of papers published by two separate groups shook the scientific world as each demonstrated the use of an artificial womb to keep mouse embryos (derived from embryonic stem cells) alive for 8.5 days. Central to both papers was a complex contraption designed to mimic the environment of the uterus, designed by Jacob Hanna, from the Weizmann Institute of Science in Israel, who led one of the teams. It combines rotating glass vials filled with blood serum and a ventilation system that maintains pressure and oxygen levels.
But the artificial womb wasn’t the only innovation. In 2018, Magdalena Zernicka-Goetz, who has dual appointments at Caltech and the University of Cambridge and led the other team, discovered that embryonic stem cells developed further when combined with stem cells from two structures key to early development: the placenta and yolk sack.
By combining these two breakthroughs, the two teams were able to create synthetic mouse embryos that developed to the stage where the basic body plan starts to emerge. This included complex structures including beating hearts, primitive brains, and the beginnings of a gut.
Game-Changing Capabilities
The most obvious impact these advanced synthetic embryos could have is helping scientists crack the underlying code of development. This underpins all kinds of critical capabilities such as regeneration, reproduction, and tissue specialization that have significant implications for medicine.
The team led by Zernicka-Goetz is particularly interested in understanding why some pregnancies fail at the earliest stages, and she says that this new model could provide an unprecedented window into these processes.
One of the beauties of this synthetic embryo model is that it should be possible to genetically engineer the stem cells used to build them. This could allow researchers to identify how specific genes contribute to the developmental process.
The other group led by Hanna has even grander ambitions. They have created a startup called Renewal Bio that plans to create synthetic embryos from patients’ cells, whose tissues could be harvested for transplantation. While there’s been significant progress in the ability to grow simple tissues like skin and cartilage, organs with complex 3D structures and vasculature have been more challenging. Hanna believes that relying on the body’s own developmental processes might be a better approach. “We view the embryo as the best 3D bioprinter,” he told MIT Technology Review. “It’s the best entity to make organs and proper tissue.”
Among the potential applications are collecting blood cells from embryos and transfusing them into patients to rejuvenate their immune systems, or using cells from rudimentary ovaries to help women extend their fertility later in life.
Ethical and Scientific Challenges
Efforts to reinvent human biology inevitably raise thorny ethical challenges. While guidelines vary among countries, human embryos are typically not allowed to develop beyond 14 days, which is the point at which they start to develop from an amorphous blob of cells into something resembling a body.
Renewal Bio envisages developing its embryos to 40-day or 50-day equivalents to gestation in pregnancies. While there is still the question of these entities being viable, the further along the developmental pathway the murkier the ethical implications. One potential solution suggested by Hanna is to genetically engineer the precursor stem cells so that the embryo never develops a brain and head, avoiding any concerns about experimenting on a potentially conscious being.
Other researchers have argued that the concerns about synthetic embryos with no potential to develop into living beings do not outweigh the concrete benefits to people on organ donor waiting lists.
But while these ethical challenges are considerable, experts say the technical ones are probably a more pressing concern. The efficiency with which stem cells can be coaxed into embryo-like structures is still very low, with less than 1% of all cell cultures doing so successfully. And even when they do, the synthetic embryos feature many defects that raise questions about whether they are truly useful models for development. And moving from mouse stem cells to human ones is not a trivial step, not least because it takes considerably longer for human embryos to reach a similar stage of development.
Final Thoughts
We remain a long way from the feasibility of the technology to combat aging or the regeneration of organs on demand, but it’s important not to understate its potential. Despite these cautious assessments of what lies ahead, experts liken these latest developments to the time when the first cloned sheep, Dolly, was born in 1997.
]]>In the past transplanted stem cells have exhibited very low survival rates. But Dr. Karimi explains what’s novel about the work being done at the University.
“We have discovered that presence of a class of inhibitory molecules in the injured spinal cord potently limits success of stem cell transplantation. When we blocked these molecules therapeutically at the time of transplantation, the treatment led to an increase in stem cell survival and generated suitable neural cells in the injured spine…This strategy enabled rats to walk with more weight support and coordination.”
I have a friend who suffered a traumatic spinal cord injury (SCI) in his teenage years and has been confined to a wheelchair ever since. The health and work challenges he has faced would make the average person curl up in a ball and check out of life. But Dan has forged ahead and created a business to help others with disabilities. He has even coached wheelchair basketball teams in the past.
In Dr. Karimi’s laboratory, the work has been led by a doctoral student, Dr. Seyed Mojtaba Hosseini, who is the lead author of a paper describing the research and results, recently published in the Journal of Neuroscience.
The paper states that SCI “is a leading cause of permanent neurologic disabilities in young adults. Functional impairments after SCI are substantially attributed to progressive neurodegeneration.”
According to the World Health Organization (WHO), between 250,000 and a half million annually suffer SCIs. The majority are caused by traffic accidents, falls or violent confrontations. The WHO notes that those who suffer from SCIs “are two to five times more likely to die prematurely than people without a spinal cord injury.” In addition, SCI victims have “lower rates of school enrollment and economic participation,” and low employment and earnings while enduring significantly higher costs of living.
At the University of Manitoba the researchers identified two receptor proteins (molecules) that inhibited the regeneration of motor and spinal interneurons. By suppressing these proteins in the laboratory in female rats, transplanted neural precursor cells (NPCs) were able to thrive and replace damaged neurons. If these results can be repeated in human patients, the treatment strategy could prove promising for neuronal replacement in SCIs.
What is the Cost of SCIs?
In Canada, in 2019, the annual cost was estimated to be $2.7 billion to cover medical management, hospitalization, and indirect costs. Long-term issues like low employment and subsidies were not factored in the data.
From U.S. data for the same year come the following numbers:
- Approximately 291,000 Americans were living with spinal cord injuries.
- 39.5% of SCIs were paraplegic (loss of the use of leg motor skills) and 59.9% were quadriplegic (loss of motor skills in all four limbs).
- Average first-year expenses for paraplegics was $550,000.
- Average first-year expenses for quadriplegics depending on where the damage occurred in the cervical spine varied from $816,000 to $1.129 million.
- Average lifetime costs for paraplegics with 25, the age of injury was $2.5 million.
- Average lifetime costs for quadriplegics with 25, the age of injury varied from $3.6 to $5 million.
- The percentage of those with SCIs who were unemployed ten years after the injury was 77%.
There is no doubt, that finding a therapy to get those with SCIs back on their feet literally would dramatically reduce lifetime costs for care, as well as give those with SCIs better prospects at long-term employment.
Dr. Karimi in the University of Manitoba press release talks about overcoming the consequences of SCIs. She states,
“Individuals with spinal cord injury live with the debilitating consequences of the disease for decades, and development of new regenerative medicine treatments is an unmet need to improve the quality of life for a large population of people.”
Further study of this approach to treatment is warranted and Dr. Karimi hopes to proceed to human clinical trials in the future.
]]>Recently I had a cardiac ablation procedure that involved inserting a catheter through my femoral vein into my heart. The ablation would correct atrial fibrillation. an arrhythmia that happened after I was infected with COVID-19. During the catheter insertion, it nicked the vein wall creating a fistula, a small connection between my femoral vein and the neighbouring artery. The complication was discovered upon examination the morning after the procedure and one of the proposed solutions from the cardiology team was to put a stent in to block the hole which would stop the shunting of blood from the artery into the vein and a potential aneurysm.
It wasn’t the first time I had encountered stents. My daughter who was born with congenital heart disease in her last heart procedure had a stent containing a valve inserted into her pulmonary artery. She was born with an absent pulmonary valve among several other heart complications.
What makes the stent developed at North Carolina State different from any that I have read about or seen in the past is the fact that it uses exosomes from stem cells as a coating. The inventors call it the Exosome-Eluting stent or EES, the cell-derived material coating on the stent when implanted in a blood vessel counters the natural inflammatory response that automatically is triggered by our bodies to repair an injury. Stent-altered vessels often end up with post-procedure narrowing, a stenosis, caused by the body’s misguided attempts at healing. It’s a self-defeating proposition when trying to open blood vessels to support heart function when a narrowing occurs resulting from treatment.
The North Carolina team led by Ke Cheng, Professor in Regenerative Medicine and Biomedical Engineering, is using mesenchymal stem cell exosome secretions to camouflage the stent so that the body’s immune defenses cannot find it. The exosome-coating also promotes the growth of endothelial cells, the normal lining in blood vessels, which form over the stent.
Up to 60% of the coating is released within 48 hours after implantation which inhibits the effect of reactive oxygen species, unstable molecules produced by our cells that cause tissue damage at a post-operative injury site.
So far the studies have been done on rats to be followed by larger animals before human clinical trials. In a North Carolina State news release, Cheng states, “This bioactive stent promotes vascular healing and ischemic repair, and a patient wouldn’t need additional procedures for regenerative therapy after the stent is in place…The stent is the perfect carrier for exosomes, and the exosomes make the stent safer and more potent in tissue repair.”
A paper describing the EES appears in an April issue of Nature Biomedical Engineering.
]]>CRISPR today is being tested in clinical trials to treat Type-1 Diabetes Mellitus, Glycogen Storage Disease, Duchenne Muscular Dystrophy, Myotonic Dystrophy, Cystic Fibrosis, and a number of refractory cancers including blood disorders and carcinomas. Add to this sickle cell anemia.
What do we know about this disease?
Today it is the most commonly inherited blood disease in the United States affecting mostly African Americans. About one in 12 carries the genetic trait, a mutation of the hemoglobin-Beta gene located on chromosome 11. That mutation causes red blood cells to deform from being circular to sickle-shaped. This makes the blood cells stiff and sticky causing clotting, organ and tissue damage. When the immune system encounters sickle-shaped blood cells they are destroyed. Hence those suffering from the genetic defect become anemic and suffer chest pains, strokes, and damage to the spleen, kidneys, and liver. They are highly vulnerable to bacterial infections.
The inherited genetic defect can be passed from parent to child. If both parents have the defect, their baby has a one in four chance of being born with the disease. If only one parent has the defect, the baby has a one in two chance of inheriting the trait and passing it along to their future children.
Using CRISPR to repair the mutation seems like a natural for the technology. And now the U.S. Food and Drug Administration has approved a clinical trial using the tool for that purpose. The planned trial is to last four years and is being led by physicians from the University of California – San Franciscio (UCSF) and University of California – Los Angeles (UCLA). The first site is the Benioff Children’s Hospital in Oakland. The Los Angeles site is the Broad Stem Cell Research Center associated with UCLA. Six adults, and three adolescents with severe sickle cell disease are the initial enrollees for the trial which will use CRISPR to snip out the defective beta-globin gene in these patients and replace them with a repaired version. If successful it will produce a cure and become a preventative so that no one need experience the irreversible complications that impact sickle cell anemia sufferers.
The trial will use each of the participants’ own hematopoietic stem cells harvested from bone marrow. These will be removed and then gene-edited and cultured. The remaining bone marrow of each participant will then be destroyed using chemotherapy. The gene-edited cells will then be reinfused into the bone marrow where they will multiply. The trial will mark its success if the genome-edited stem cells correct the mutation enough to impact the red blood cell population. A correction in 20% of the bone marrow should be sufficient to knock out the participants’ sickle cells.
In future, the hope is to create a technique for correcting the sickle cell mutation without removing the stem cells or destroying bone marrow because this would shorten the amount of time the participants are immune-compromised and subject to the potential complications that come with the use of strong chemotherapy drugs. This could be accomplished using a nanoparticle delivery of the CRISPR enzyme directly to the stem cells.
If the current four-year trial is successful it will open the door to treating all types of blood disorders using a similar modality. And ultimately if the treatment can be delivered directly to stem cells within the body, it will be revolutionary.
]]>I’m not a Kentucky Fried Chicken (KFC) afficionado. But imagine if KFC were to produce its chicken nuggets from stem cells and 3D-printing plants. In 2020 the news wires lit up with stories of a Moscow, Russia, research laboratory under contract to the fried chicken restaurant chain to produce 3D-printed chicken nuggets.
For KFC the announcement could be seen as a public relations coup since the company is often the target of animal rights advocacy groups. KFC is truly a global enterprise, found in 145 countries at 24,000 individual locations. According to PETA, an organization focused on the ethical treatment of animals, 9 billion chickens raised on factory farms are slaughtered for their meat in the U.S. every year. A good percentage of that number go to fast-food chains like KFC.
That’s why KFC sees the growing of meat harvested from cell-cultures as a way out of the ethical dilemma. A future where the restauranteur can say “no chickens were killed here” would be a welcome mantra with other potential benefits to the global enivronment.
So how does cultured meat work?
This is cellular agriculture. Its products are called cultured meat. The source of cultured meat is animal stem cells harvested from subject hosts that are not slaughtered. Once ideal chicken, pig, sheep, cattle, etc., candidates are identified, stem cells are harvested and then using electronic, chemical and biological culturing cultivated to create vast populations of cells of various tissue types from muscle to fat.
Turning stem cells from host animals into chicken pieces, beef steaks, pork and lamb chops, and other cuts of meat requires scaffoldings of bio-absorbable materials which form a framework for 3D printers to apply these cells as “ink” to create finished cuts. Getting the balance of fat to protein to give the 3D-printed meat the same look, texture, and taste is a challenge that the technology in time can meet.
Is KFC actually serving 3D-printed chicken nuggets in any test markets presently?
The company has produced plant-based “chicken” nuggets and tried them on customers in the United States using Beyond Meats’ chicken products. But, as for 3D-printed chicken, it doesn’t appear that, as of yet, the product is ready for prime time testing. In the summer of 2020, the announcement suggested field testing would start in the fall at KFC outlets in Moscow. But my online search has uncovered nothing to support whether field trials have begun. And who knows, the COVID-19 pandemic may have temporarily stopped the development and rollout of 3D-printed chicken nuggets.
What are the benefits of using cell-cultured versus livestock-derived meat?
Besides the animal lives saved, a number of studies cite energy savings, greenhouse gas emission reductions, and redeployment of land as the prime benefits of cell-cultured meat. But some question the environmental gains stating that cultured meat could end up yielding more greenhouse gas emissions from the factories responsible for its production than current animal agricultural practices which contribute an estimated 14% of all global emissions.
Those arguing the benefits of cultured meat in a 2011 study looked at beef, sheep, pork, and poultry production and calculated the following:
- Cultured meat production would produce between 78 and 96% less greenhouse gas emissions.
- Cultured meat production would use 99% less land.
- Cultured meat production would use 82 to 96% less water.
- And cultured meat would require 7 to 45% less energy.
The study noted that of all traditional livestock agriculture practices, the raising of poultry in factory farms has the smallest environmental footprint. Having said that, cultured poultry would have a far smaller environmental impact than any current methods of producing chicken for human consumption.
What are the inhibitors to switching to cell-cultured meat?
The biggest one at present is the cost. In May 2014 I posted an article to this site that described the first lab-grown hamburger taste test. The cost to make a single hamburger patty was $325,000 U.S.
Shortly after this hamburger’s debut, a company announced its plans to develop cell-cultured meat products as an alternative to livestock-produced meat and estimated it would take it at least 5 years to get its production costs down to where consumers could afford it. That has yet to happen but no doubt, in the face of climate change concerns, and the growing worldwide demand for meat in everyday diets, that new disruptive technology will begin to displace traditional animal husbandry in agriculture within this decade. That KFC bucket you order in the future may not have harmed a single chicken
]]>
June 11, 2020 – About a week ago I received an e-mail blast from Peter Diamandis, of X-Prize fame, in which he stated that the world is facing two pandemics: the first COVID-19, the second, aging. Aging has been with us as a species from the moment humans emerged from the tree of life. Calling it a pandemic seems facile. It’s like calling erectile dysfunction a disease in men rather than a sign of aging. But nonetheless, the biopharmaceutical industry and scientific community are tackling aging as if it were a disease.
In Peter’s e-mail he reviewed the technologies on the cutting edge that could add 20 to 30 healthy years to the lifespan of the average human. He describes 100 as the new 60 in the coming decades.
On this planet today live 7.8 billion humans of which 9% are over the age of 65. That’s approximately 720 million of us who have reached a point where the accumulations of our life’s journey has piled up leaving us with chronic health problems like heart and respiratory diseases, cancer, arthritis, dementia, and other conditions. These problems don’t start the day we turn 65. They are acquired over a lifetime and some believe they are symptoms of larger condition called aging. Peter argues that science and medicine is only now beginning to recognize the spectrum of conditions as being within the overall disease called aging.
Should we accept the natural limits of our species? And if we do, just what are these limits? Is it 90, 100, 110, or more? Many species on this planet outlive us. Bowhead whales can live for 200 years, and Greenland Sharks, Sea Turtles, and some land tortoises can make it to 400 years or more. And then there are trees that can live thousands of years. What is innate in them that is not in us? Peter asks if this is a hardware or software problem, and if so, don’t we already have sufficient knowledge and the tools necessary to repair both?
On this blog site we write about some of these tools such as CRISPR, stem cells, gene drives, and more. Dr. David Sinclair of Harvard Medical School, in his book Lifespan, believes the technologies we have or are in the process of maturing will allow people born today to live to 120 in good health, and even make it to 150 or more. These tools include:
- CRISPR & Gene Therapy
- Stem Cell Therapy
- Wnt Pathway Manipulation
- Senolytic Medicines
CRISPR & Gene Therapy
Aging happens because our normal cell functions over a lifetime begin to destabilize. When cells no longer have the capacity to thrive they experience apoptosis, cell death. But in using gene therapy tools there is no reason for specific cells in our bodies to die. And with CRISPR-Cas9, we can go into our DNA and edit it inside the cell to provide a repair kit for almost any problem.
Today we can re-engineer our DNA instructions by target specific areas of the genome, snipping out bad code, and inserting a healthy bit of replacement instructions. CRISPR is cheap, fast, and getting easier to use. Recently, scientists at the Broad Institute associated with MIT and Harvard University, unveiled CRISPR 2.0, a next-generation editor that’s extremely precise capable of changing a single nucleobase of which there are four strung out along the double helix that is the basis for life on this planet.
David Liu, a Harvard chemical biologist states, “Of more than 50,000 genetic changes currently known to be associated with disease in humans … 32,000 of those are caused by the simple swap of one base pair for another.”
Stem Cell Therapy
We are made up of more than 30 trillion cells. All of them were derived from the embryo defined as pluripotent stem cells. Our bodies contain millions of stem cells which can replace damaged tissue. As we age the number of stem cells decline.
But what if we could restore our stem cell population? What if we could take stem cells from harvested placentas and augment our stem cell population? Celularity is a company doing just this to extend life using placenta-derived stem cells.
Wnt Pathway Manipulation
A San Diego-based company, Samumed, is targeting the signaling pathways that regulate adult stem cell self-renewal and differentiation through a pathway known as “Wnt” which stands for Wingless and Int-1, pathways to allow proteins that engage the surface receptors on the cell wall to pass signals to the cell’s inner workings. Proteins are critical to executing DNA and RNA instructions and Samumed is using this normal cellular process to deliver instructions through nine new drugs to regrow cartilage, heal tendons, remove wrinkles, stop a multitude of cancers, and reverse Alzheimer’s.
Senolytic Medicines
Our body’s cells have a limited shelf life. They can divide approximately 50 times before dying, a condition called apoptosis. But what if we could end programmed cellular death without unintended consequences?
In our body today a small fraction of our cells are senescent cells that don’t go through an end of life with damaging consequences for our bodies. These cells secrete molecules that trigger inflammatory responses and change the behaviour of nearby cells. They are associated with many of the symptoms of aging including fibrosis, blood vessel calcification, osteoarthritis, and diminished organ function.
Unity Biotechnology is a Brisbane, California company focused on developing senolytic therapies with a goal to eliminate senescent cells associated with osteoarthritis, pulmonary diseases, and other aging conditions. They have under development a number of senolytic medications aimed at musculoskeletal, ophthalmology, pulmonary, neurology, and multiple organ aging challenges.
One of their drugs under development, UBX0101, is being evaluated for the treatment of osteoarthritis of the knee. That got me interested. ApparentlyUBX0101 is a small molecule inhibitor of the MDM2/p53 protein to trigger the elimination of senescent cells. In Phase 1 clinical trial, with patients suffering from moderate to severe osteoarthritis of the knee, initial results announced in June of last year showed promising improvements in pain and function. That’s not a cure, but for someone suffering from osteoarthritis of the knee, those two improvements would be a welcome relief.
